SCPC

ShefaBone SCPC™ Silica-Calcium Phosphate Composite Bone Graft Granules
Structure of SCPC
Mineralization and Vascularization of De Novo Bone
Cell-Mediated Resorption of SCPC
Advantages of SCPC
Product Information
Structure of SCPC
Shefabone SCPC is a US patented, FDA approved resorbable osteoconductive bone graft material made of bioactive silica-calcium phosphate ceramic [1, 2]. SCPC is engineered with unique interconnected pores that expedite new bone formation, vascularization, and graft material resorption.
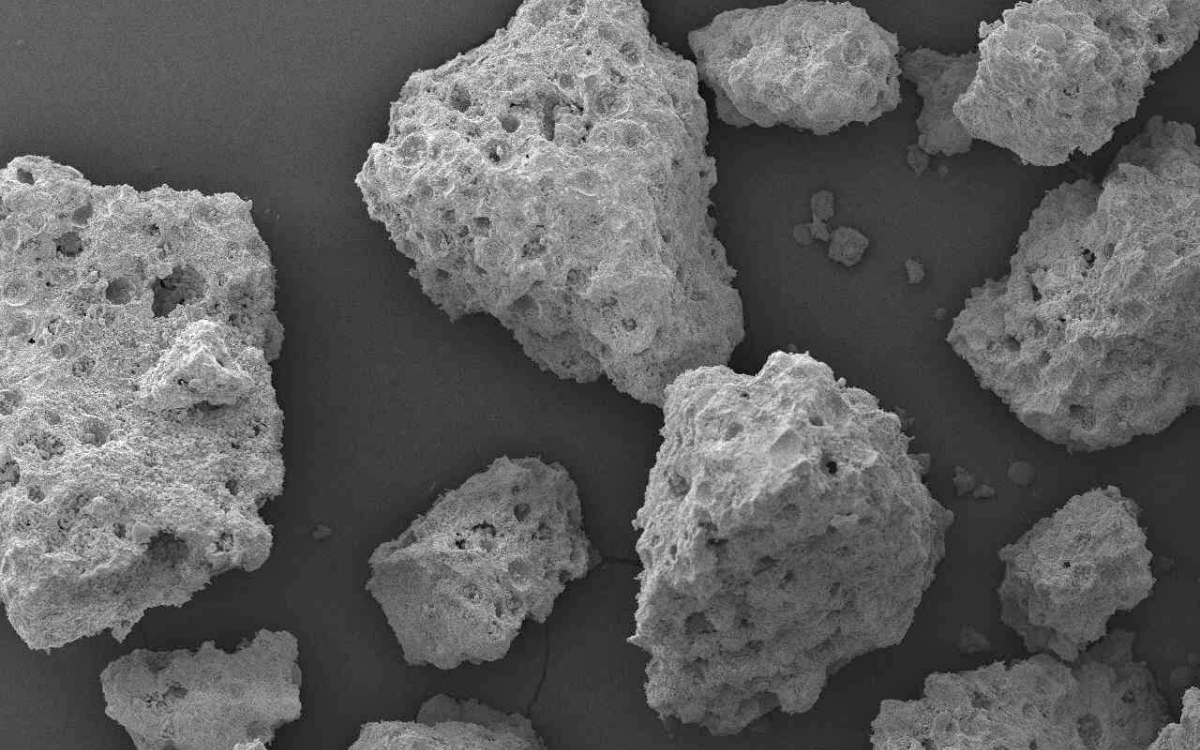
Figure. 1. Scanning electron microscope showing the porous structure of the SCPC granules.
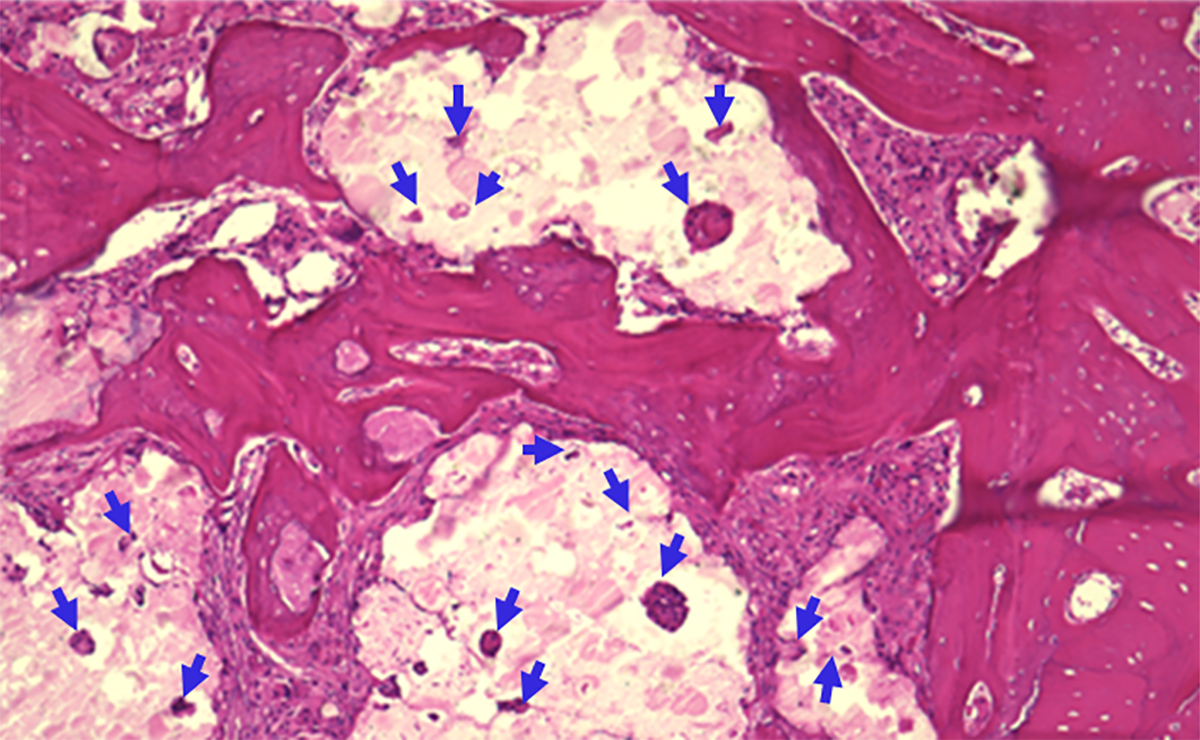
Figure 2. Histology analysis of a critical size calvarial defect grafted with SCPC granules for 4 weeks. New bone formation in the interspace between SCPC particles as well as within the pores of SCPC is noticed. The arrows point to the bone formation inside the pores of SCPC granules.
Mineralization and Vascularization of De Novo Bone
After implantation, the SCPC develops a carbonate hydroxyapatite surface layer, similar to the mineral phase of bone and selectively adsorbs attachment proteins from serum which facilitates osteoblasts attachment, proliferation, differentiation and new bone formation.
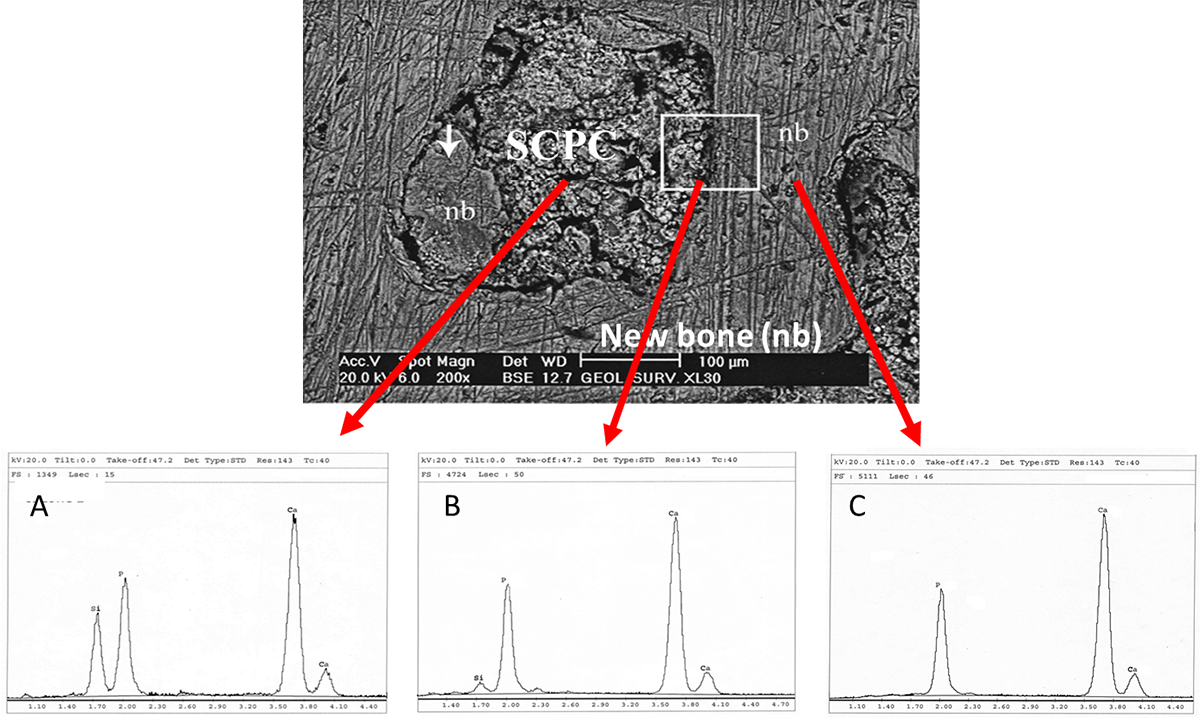
Figure. 3 (Upper) Back scattered electron microscope image of non decalcified section inside rabbit femoral bone defect grafted with SCPC granules for 8 weeks. Mineralized hard bone can be seen around the SCPC particles inside the grafted defect. Bone formation was deposited directly on the surface of the graft material. (Lower panel) Energy Dispersive X-ray elemental (EDX) analysis of (A) the SCPC particle, (B) the SCPC/bone interface and (C) the new bone. (A) The EDX spectrum of SCPC granule showed a relatively small signal for Si and absence of any signal for Na due to resorption of the graft material. (B) The surface of the SCPC granule showed high signals of Ca and P indicating formation of a calcium phosphate rich layer on the SCPC surface. (C) The newly formed bone has a Ca/P ratio = 1.475 typical for biological hydroxyapatite mineralization.
ShefaBone SCPC™ granules recruit osteoprogenitor cells, activate osteogenic gene expression and provide differentiated osteoblasts with calcium and phosphorus ions essential for new bone formation. The SCPC granules release silicate ions known to promote the metabolic activity of bone cells, enhance collagen type I production and calcified nodules formation. Most importantly silicate ions facilitate vascularization of the newly formed bone. The vascularization of the newly formed bone is essential for maintaining the viability and functionality of the new bone.
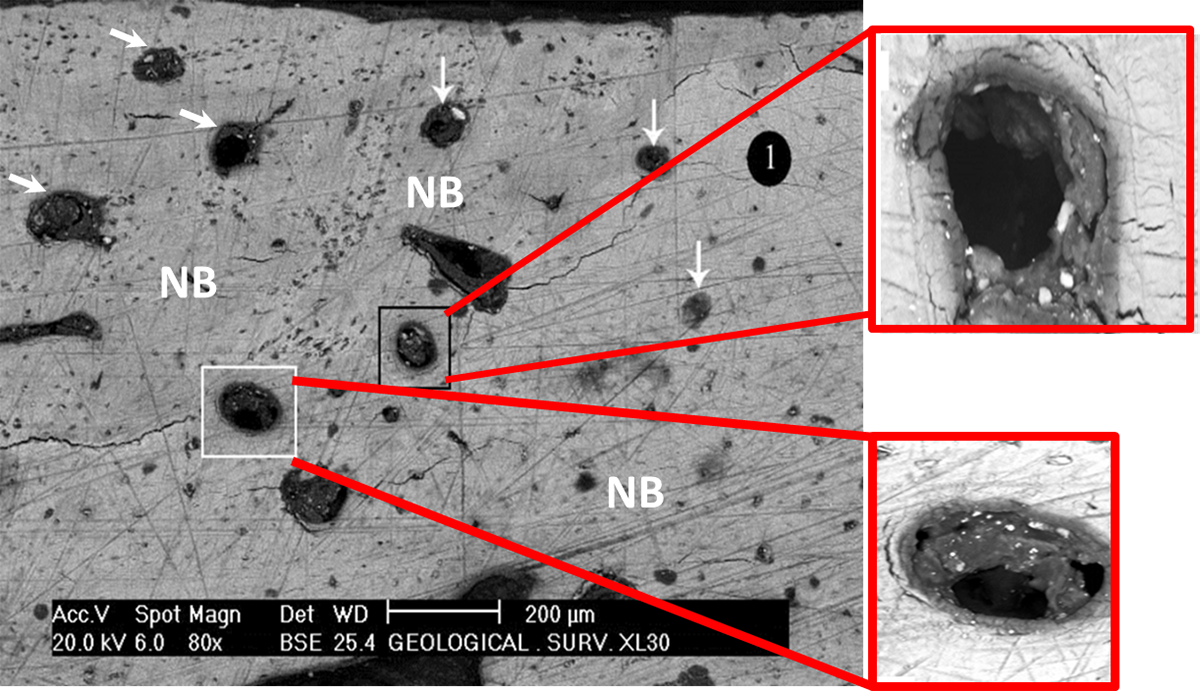
Figure 4. Back scattered scanning electron microscope image of a non decalcified section of rabbit femoral defect grafted with SCPC granules for 16 weeks. All the SCPC granules were completely resorbed and replaced by new mature strong bone (NB). The newly formed bone was completely remodeled lamellar bone; with Haversian systems and architecture that resemble healthy functioning bone. The arrows and the black and white boxes were magnified to show the morphology of the Haversian system and blood vessels.
Cell-mediated resorption of SCPC
The resorption of SCPC is cell mediated indicating that the rate of new bone formation matches the rate of graft material resorption. This is a major advantage over other graft materials that resorb quickly by dissolution and leave empty space inside the defect. Bone cells absorb calcium and phosphorous ions from SCPC and use them to produce new mineralized bone tissue. As the SCPC resorbs, bone cells deposit new bone that fully replaces the graft material. There is no fibrous encapsulation or immune reaction against SCPC (Figure 5).
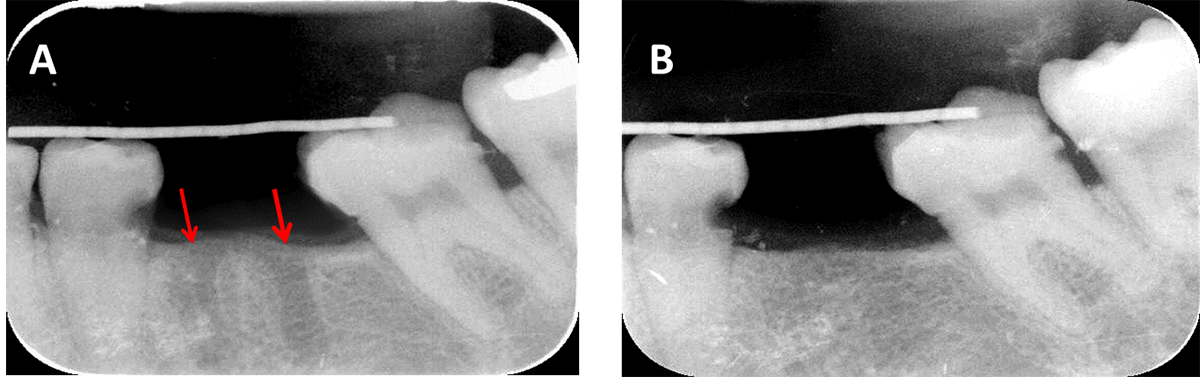
Figure 5. X-ray radiographs showing: (A) immediate postoperative image of the extraction sockets (red arrows) and (B) after 6 months, the SCPC granules were fully resorbed and replaced by new bone which has comparable radio-opacity to the host bone.
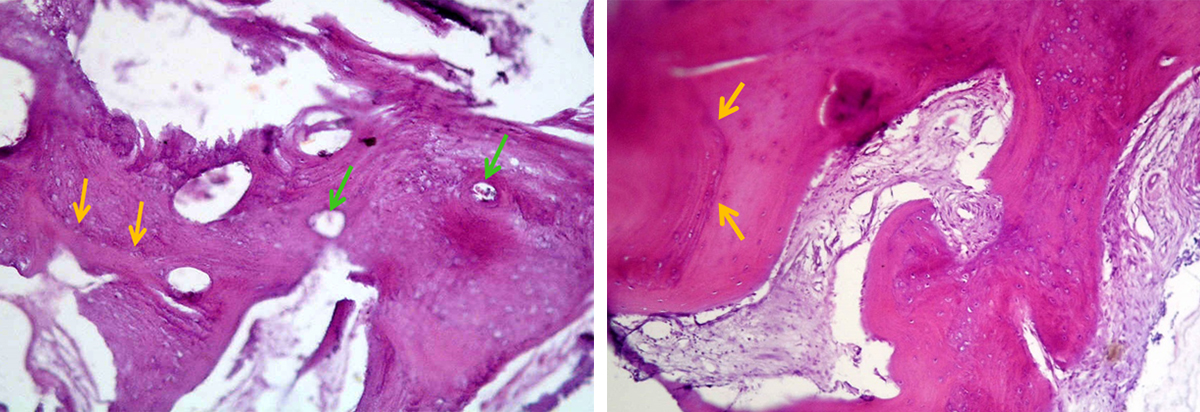
Figure 6. Histology analysis of human bone biopsy taken from the core of extraction socket grafted with SCPC (for 6 months) at the time of implant insertion. Areas of mature bone with osteons and Haversian system are seen. The resting and reversal lines (yellow arrows) can be seen together with wide thin walled capillaries (green arrows). Areas with woven bone are also present. Sample stained with H&E; magnification 100X.
Bone formation in the inner pores and on the outer surface of SCPC granules accelerates regeneration of functioning bone made by the patient own cells. The newly formed bone undergoes remodeling exactly like host bone. SCPC has successfully passed all ISO 10993-1 biocompatibility tests and showed complete absence of sensitization, irritation, systemic toxicity, and genotoxicity.
Advantages of SCPC
- Bioactive: Stimulates bone cell function and tissue formation
- Osteoconductive: Enhances bone cells differentiation and directs bone deposition on the material surface.
- Porous: Allows rapid bone formation, vascularization and graft material resorption
- Resorbable: Allows complete bone regeneration as the body fully resorbs the graft material.
- Handling: Easy to apply, SCPC particles stay in place inside the defect and do not migrate or diffuse.
- Biocompatibility: Nontoxic, non-immunogenic and does not elicit any rejection reaction
- Efficiency: Minimizes the need for autogenous bone in large defects after tumor resection
- Radiopacity: SCPC is radiopaque in X-ray radiograph which makes it easy to follow the progress in bone healing and graft material resorption.
Why is SCPC bone graft better than other silicate bioactive glasses (BG)??
SCPC granules have a stronger stimulatory effect on new bone formation and they are fully resorbed in a rate that matches that of new bone formation. Moreover, the porosity of SCPC allows bone formation within the pores of the granules which accelerates bone formation and graft material resorption.
Histological analyses showed that the bioactivity and resorbability of SCPC is superior to those of bioactive glass (BG) (Figure 5). BG particles are not porous which limits bone formation to the outer surface of the material. Moreover, BG particles form a carbonate hydroxyapatite layer on the top of a silica-rich layer thus bone cells have limited access to the stimulatory effect of the silicate ions. The formation of a silica rich layer slows down BG dissolution by inhibiting Ca, P and Si ion diffusion from the glass bulk into the surface [6]. The implication for the slow rate of dissolution of BG is the slow rate of resorption inside bone defects. While the resorption and bioactivity of BG are restricted by ion diffusion from the glass bulk to the surface, the resorption and bioactivity of the SCPC are enhanced by the porous structure and the chemical reactivity of the modified crystalline phases [1-5].
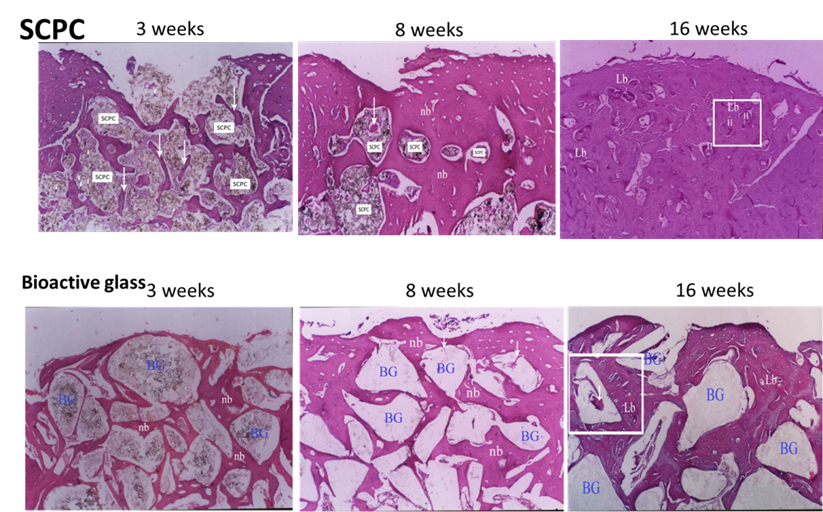
Figure 7. Histology analysis of a rabbit femoral critical size bone defect grafted with SCPC or bioactive glass (BG) granules for 3, 8 and 16 weeks. A successful regeneration of the cortical bone and SCPC graft resorption was observed after 16 weeks. The newly formed bone completely remodeled to a mature lamellar bone showing restoration of the Haversian systems (H). On the other hand, BG particles occupied close to 50% of the bone defect area after 16 weeks due to the slow rate of resorption. (H&E staining, original magnification x40).
Figure 5. Histology analysis of a rabbit femoral critical size bone defect grafted with SCPC or bioactive glass (BG) granules for 3, 8 and 16 weeks. After 16 weeks, complete regeneration of the cortical bone and full resorption of the SCPC granules were observed. The newly formed bone completely remodeled to a mature lamellar bone showing restoration of the Haversian systems (H). On the other hand, BG particles grafted in bone for 16 weeks did not resorb and occupied close to 50% of the bone defect area. (H&E staining, original magnification x40).
X-ray radiography confirmed the histology analyses and showed the resorption of SCPC graft and restoration of the continuity of the cortical bone after 16 weeks of implantation (Figure 6). For defects grafted with BG particles, X-ray radiography showed restoration of the continuity of the cortical defect, however, the presence of significant amount of BG particles in the defect is evident after 16 weeks.
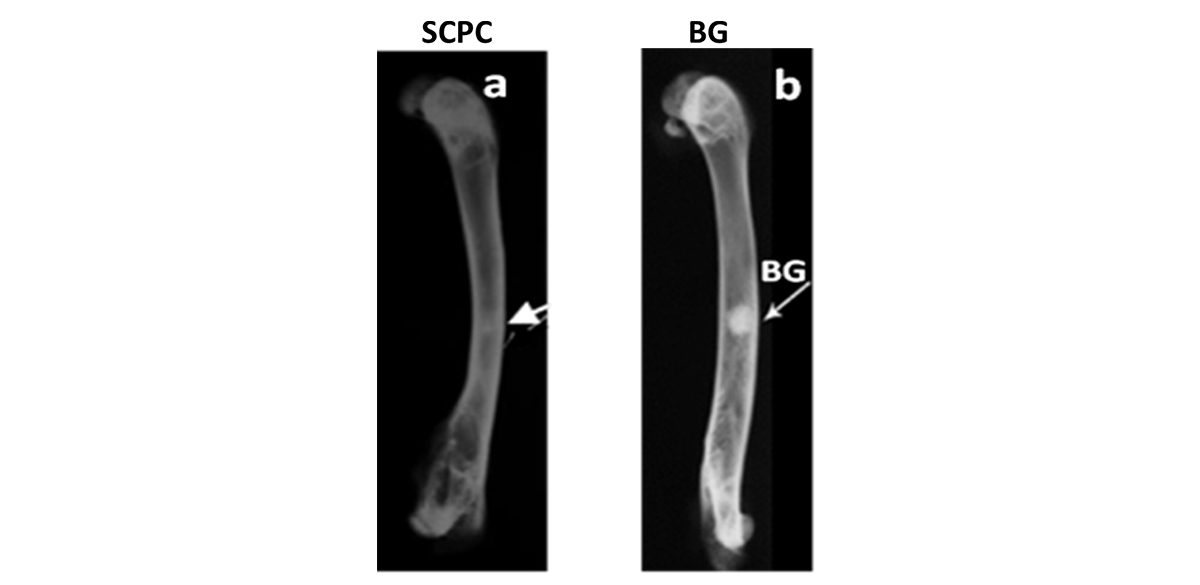
Figure 8. X-ray radiography of rabbit femoral bone defect grafted with (a) SCPC granules and (b) BG particles; 16 weeks postoperative. SCPC-grafted bone defect showed complete restoration of the cortical continuity and graft material resorption. On the other hand, a significant amount of BG granules remained un-resorbed in the defect after the same time period.
Why is SCPC better than other calcium phosphate bone grafting materials?
The incorporation of silicate in SCPC structure is considered a major advantage over bone grafts made of calcium phosphate ceramics due to its important role in the upregulation of osteoblastic cells and development of osteoid and mineralized skeleton [10]. Moreover, the main calcium phosphate phase in SCPC is NaCaPO4 which possesses a higher bioactivity than tricalcium phosphate (TCP) and other calcium phosphates, thereby promoting osteogenesis and bone matrix calcification [7]. Although evidence of bone growth in porous and dense hydroxyapatite (HA) particulates is widespread, the bone conductive effect is limited [8]. Often HA particulates are encapsulated by fibrous tissue. TCP is more biodegradable than HA; however, its presence elicits a foreign body response [9]. Biological bone grafts such as cadaver bone have many limitations related to rejection reaction due to genetic differences or disease transmission. With SCPC being a synthetic resorbable bioactive bone graft there is no need to address issues related to problems involving allergic reactions or the potential risks of infection transmission from materials like bovine bone, processed bone matrix or cadaveric demineralized freeze-dried bone allograft.
Product Information

Indications of Use
ShefaBone SCPC Resorbable Bioactive granules are used as a bone filling material in orthopedic and maxillofacial surgeries. Typical uses include:
- Cystic cavities
- Periodontal / infrabony defects
- Ridge augmentation (sinusotomy, osteotomy, cystectomy)
- Extraction sites (ridge maintenance/augmentation, implant preparation/placement)
- Sinus lifts
- Oral and maxillofacial augmentation
These instructions are intended as guidelines for the use of ShefaBone SCPC as a part of established surgical techniques. They are not intended to replace or change standard procedures for treatment of bone defects involving bone grafting and internal fixation. The seal on the vial should be carefully removed to avoid spillage. ShefaBone SCPC granules can be mixed with the patient’s blood or sterile saline using a disposable dappen dish cup and subsequently delivered to the defect site with a spatula. Allow material to hydrate in the bone defect. Gently pack the particles and do not over fill. The granules in the bone defect can be covered by the soft tissue and suture or a resorbable collagen plug (RCP), or tape (RCT), can be cut to size and compressed in place as a dressing (cover the SCPC particles inside the socket or bone defect). Criss-cross and/or mattress sutures are placed. Allow to heal completely.
Content per vial: Each vial contains 1 cc of resorbable porous bioactive SCPC granules.
Sterilization
Sterilized by Gamma radiation. Contents are sterile if unopened and undamaged.
SINGLE Use ONLY. Do not attempt to re-sterilize or re-use
Contraindications
ShefaBone SCPC should not be used in patients in which general oral surgery is not advisable.
Caution
℞ Only. ShefaBone SCPC is intended for use on the order of a physician or dentist.
Precautions
ShefaBone SCPC should not be used outside its intended indications. Underlying oral pathological conditions, such as infections, should be controlled or eliminated prior to implementation of ShefaBone SCPC. Care should be exercised to avoid salivary contamination. ShefaBone SCPC vial is for Single Use Only. Reuse of products that are labeled for single use may result in product contamination, patient infection and/or failure of the device to perform as intended. Standard postoperative practices for the treatment and rehabilitation associated with bone grafting must be strictly followed.
Warnings
- Content of package is sterile unless opened or damaged. Do not use if package is opened or damaged or if expiration date has been exceeded
- Dose is SINGLE USE ONLY. Do not attempt to re-sterilize or re-use.
- SCPC does not possess sufficient mechanical strength to support load bearing defects prior to hard tissue ingrowth. In cases of fracture fixation or where load support is required, standard internal or external stabilization techniques must be followed to obtain rigid stabilization in all planes.
- SCPC is intended for use by a clinician familiar with bone grafting and internal/external fixation techniques.
- SCPC must not be used to gain screw purchase or to stabilize screw placement.
- Not intended for immediate load-bearing (allow approximately 16 weeks for substantial bone reconstruction)
- Do not overfill defects
- Do not leave defect open
- Do not compromise blood supply to the defect area
- The device should be secured to prevent motion and migration, use in areas where the graft can be adequately contained
References
- El-Ghannam A. “Resorbable, Porous, Silica-Calcium Phosphate Bioactive Composite For Improved Synthetic Graft Resorbability and Tissue Regeneration”, US patent number 8168208B1 (2012)
- El-Ghannam A., “Resorbable, Porous, Silica-Calcium Phosphate Bioactive Composite For Improved Synthetic Graft Resorbability and Tissue Regeneration”, US patent No. 7,223,414B1 (2007)
- Fahmy RA, Mahmoud N, Soliman S, Nouh SR, Cunningham L, El-Ghannam A., Acceleration of Alveolar Ridge Augmentation Using a Low Dose of rhBMP2 Loaded on a Resorbable Bioactive Ceramic, J Oral Maxillofac Surg. 2015 Dec;73(12):2257-72.
- Mohanad Al-Sabbagh, John Burt, Ahmed Barakat, Ahmad Kutkut and Ahmed El-Ghannam, “Alveolar Ridge Preservation Using Resorbable Bioactive Ceramic Composite: A histological Study”, Journal of the International Academy of Periodontology, Journal of the International Academy of Periodontology 15/3:91-98 (2013)
- Ahmed El-Ghannam, “Advanced Bioceramic Composite for Bone Tissue Engineering: Design Principles and Structure-Bioactivity Relationship”, Journal of Biomedical Materials Research, 1;69A(3):490-501 (2004)
- L. Hench “Bioceramcis: From Concepts to Clinic” J. Amer. Ceram Soc., 74[7] (1991).
- M. Knotts, S. Jalota, S.B. Bhaduri and A. C. Tas. “Synthesis of Rhenanite (B-NaCaPO4)-Apatititc calcium phosphate biphasics for skeletal repair”. Advances in bioceramics and porous ceramics, Ceramics Engineering and Science Proceedings, editors: R Narayan and P. Colombo, John Wiley & Sons publication, vol 29, issue 7, p. 152, 2008.
- Schepers E, de Clercq M, Ducheyne P, Kempeneers R. Bioactive glass particulate material as a filler for bone lesions. J Oral Rehabil 1991; 18:439–445.
- Eggli PS, Muller W, Schenk RK. Porous hydroxyapatite and tricalcium phosphate cylinders with two different pore size ranges implanted in the cancellous bone of rabbits. Clin Orthop 1988; 232:127–137.
Jugdaohsingh, Silicon and Bone Health, J Nutr Health Aging, 2007 ; 11(2): 99–110
